
Southworth Mary Ross, Reichman Marsha E., Unger Ellis F., Dabigatran and Postmarketing Reports of Bleeding, 10.1056/nejmp1302834.Sholzberg Michelle, Pavenski Katerina, Shehata Nadine, Cserti-Gazdewich Christine, Lin Yulia, Bleeding complications from the direct oral anticoagulants, 10.1186/s1287-z.

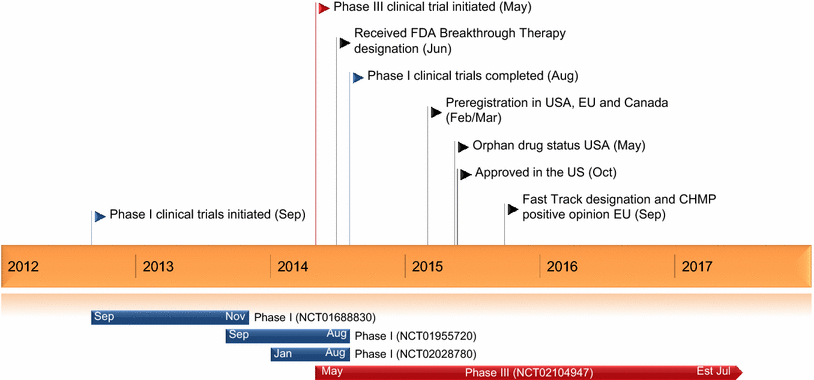
Furthermore, the benefit of idarucizumab in specific indications such as acute ischemic stroke should be investigated. Idarucizumab should be integrated into protocol for the emergency management of patients on DE. Information regarding the clinical context in which patients on DE are admitted for emergency treatment, and accurate laboratory tests of dabigatran plasma level during reversal may inform selection and help with the follow-up of patients who may benefit from idarucizumab. Expert opinion: Although idarucizumab has shown clear efficacy in reversing dabigatran-induced coagulopathy, its overall effects on patient outcome have not been proven. The results of the clinical phase III trial RE-VERSE AD and a review of recent case reports of idarucizumab use in emergency contexts are also discussed. Areas covered: The authors provide an overview of idarucizumab development and its pharmacokinetic and pharmacodynamic properties. However, idarucizumab use should be limited to specific indications to avoid unnecessary risks to patients and costs to healthcare systems. The recent market authorization of idarucizumab in Europe and the USA may reassure prescribers of DE, as it can increase the safety of the emergency management of patients taking this anticoagulant. Idarucizumab is a specific antagonist for dabigatran etexilate (DE).


 0 kommentar(er)
0 kommentar(er)
